ENROLLMENT VS PRIMARY ADJUDICATED CATEGORY
GOAL:
This document outlines the categorization of KPMP participants following enrollment and adjudication.
The goal of the KPMP Adjudication Committee is to review participants enrolled in KPMP and classify them into kidney disease categories that are considered the primary cause of the participant’s kidney disease based on clinical and pathological data. Additional causes noted in adjudication are available in the Core Clinical File and can be made available with a signed Data Use Agreement (DUA).
CATEGORIZATION:
Each participant is sub-categorized in different cohorts or disease classes at different steps of assessment
- Enrollment categories - participants enter the study classified in the following broad categories prior to kidney biopsy. Recruitment site investigators and research coordinators use study inclusion/exclusion criteria to determine the participant’s enrollment category. Refer to the KPMP protocol for more details on inclusion criteria.
- Healthy Reference: participant donating a kidney or undergoing treatment for kidney stones
- Chronic Kidney Disease (CKD): diagnosis of diabetes OR hypertension (based on inclusion criteria) AND evidence of persistent kidney damage (based on inclusion criteria)
- Acute Kidney Injury (AKI): evidence of acute kidney injury (based on inclusion criteria)
- Diabetes Mellitus Resilience (DM-R): participant has had diabetes for more than 25 years and has no clinical signs of kidney disease (based on inclusion criteria)
- Primary Adjudicated categories - upon review of the clinical and pathologic characteristics, participants are categorized using primary and secondary disease categories. The primary disease category reflects a consensus of pathologists and nephrologists on the primary cause of the participant’s kidney disease. The Primary Adjudicated categories are the following:
- Acute Interstitial Nephritis: inflammatory infiltration into the tubulointerstitium considered to be caused by drug exposure or other etiologies
- Acute Tubular Injury: clinical and histologic features of acute tubular injury
- Cannot be determined: the pattern of injury did not fit any known kidney disease agreed upon by the adjudication committee or the predominant pattern of injury could not be determined amongst several plausible underlying kidney diseases, or there was not enough information (clinical or limited tissue sample)
- Diabetic Kidney Disease: histologic features consistent with diabetic kidney disease in an individual with a history of diabetes
- Hypertensive Kidney Disease: histologic features consistent with hypertensive kidney disease in an individual with a history of hypertension
- Other: another kidney condition, including IgA nephropathy, focal segmental glomerulosclerosis, membranous nephropathy, fibrillary glomerulonephritis, etc. More specific diagnoses are available for release with a DUA.
ADJUDICATION PROCESS:
The Adjudication Team at KPMP comprises a group of Pathologists and Clinicians. Each participant’s case is reviewed with the following information:
- Whole Slide Images in Digital Pathology Repository (DPR)
- Clinical Packet: REDCap participant reported medical history, medications, and lab data completed by the recruitment site
- Pathology Packet: Diagnostic core disease category assessment REDCap form
- Electron Microscopy (EM) Images
- Recruitment Site Pathologist generated pathology report
Upon review of the materials, the KPMP adjudicators (clinicians and pathologists) meet and reach a consensus of primary and secondary categories and record in KPMP’s REDCAP Adjudication form.
Data is available in our Core Clinical File and is accessible with a DUA.
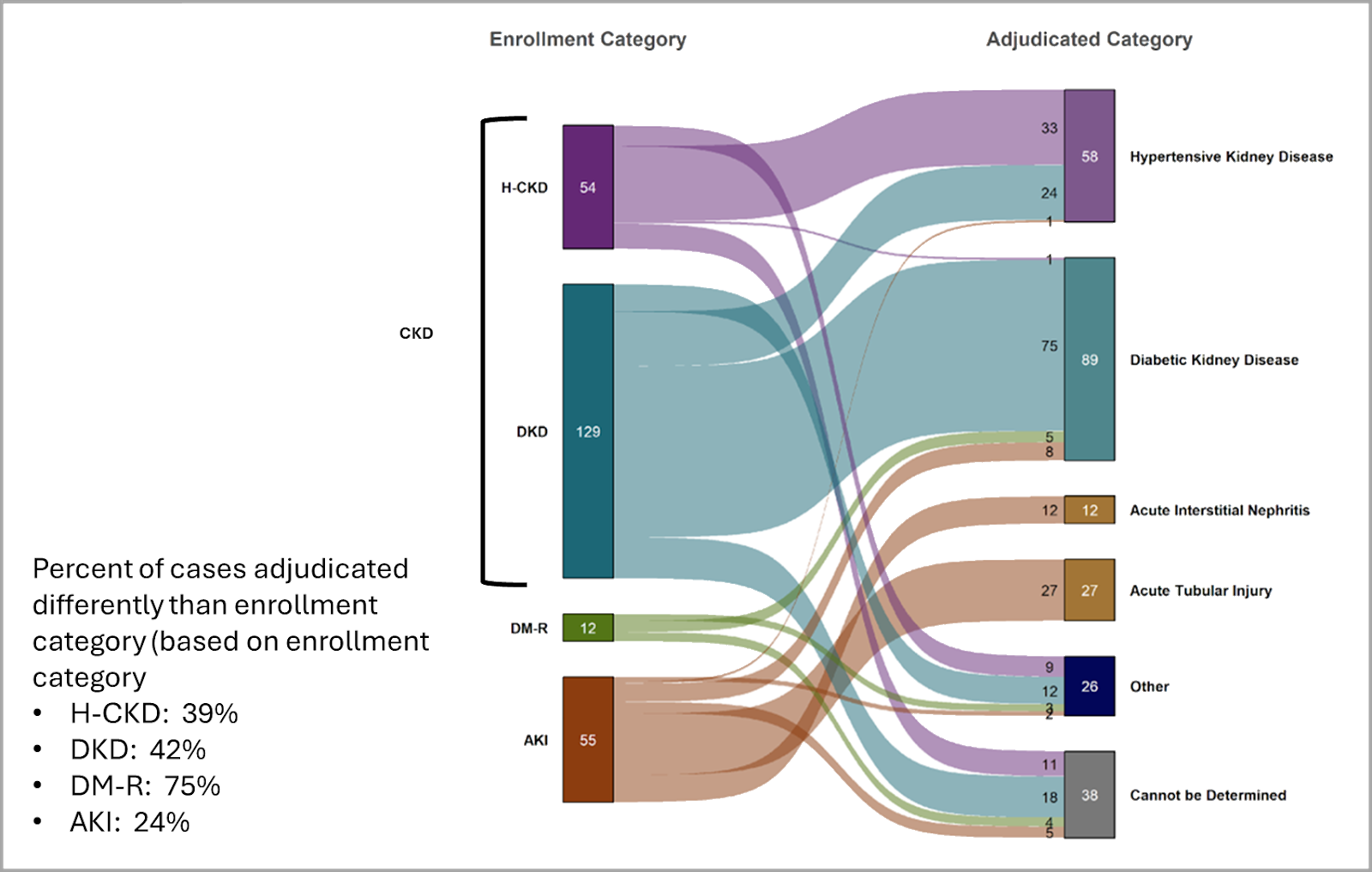
ENROLLMENT VS. PRIMARY ADJUDICATION:
The KPMP Enrollment Category reflects how each participant is enrolled in the KPMP study prior to biopsy based on inclusion criteria. For more details on the KPMP study criteria, please visit our Clinician Resources page. KPMP biopsies of participants enrolled in the CKD, AKI, or DM-R cohorts (“Enrollment categories”) are adjudicated through a comprehensive review of the participant’s clinical history and kidney biopsy pathology by expert nephrologists and renal pathologists on a consensus basis (“Primary Adjudicated categories”). Enrollment category and Primary Adjudicated category may differ.
For example, take a participant presenting with AKI enrolled in the AKI enrollment category. Upon adjudication:
- Clinical history reveals a long-standing history of hypertension and diabetes.
- Pathology review shows significant diabetic, global, and segmental glomerulosclerosis and severe arteriolosclerosis with moderate arteriolar hyalinosis. While mild to moderate tubular and interstitial injury in this case is present, and therefore the enrollment category is correct, the chronic damage and long-standing clinical history are more notable. The adjudication team ascertains that the primary driver of this participant’s kidney disease is diabetic kidney disease.
- This participant would have an Enrollment category of AKI and a Primary Adjudicated category of Diabetic Kidney Disease.
This adjudication process ensures a more accurate classification of kidney disease by integrating both clinical and pathologic insights, which is crucial for research, treatment decisions, and understanding disease progression.
Figure 1 demonstrates that the Enrollment category and Primary Adjudicated category do not always align.
Introduction
The Kidney Precision Medicine Project (KPMP) is a prospective cohort study, whose goal is to use deep molecular phenotypes of kidney biopsies, along with longitudinally collected clinical phenotypic data, in order to develop new disease ontologies, classification systems, and treatments for acute kidney injury (AKI) and chronic kidney disease (CKD).
For each participant, kidney tissue is obtained for molecular phenotyping and clinical diagnosis. In addition to the kidney biopsy tissue, the study collects baseline (time of biopsy) and longitudinal biospecimens (including urine, plasma, serum, DNA, and stool) and demographic, clinical, and laboratory data.
Participant Population
The KPMP is focusing on participants with acute and chronic kidney disease, who account for a large portion of the public health concern as reported by research and federal data.
CKD
- High priority populations include CKD in the setting of diabetes (diabetic kidney disease, DKD) and hypertension-associated CKD (H-CKD).
DM-R
- A special population of people with long-standing type 1 diabetes (more than 25 years) who remain free of clinically-evident DKD (i.e. diabetes mellitus-resilient (DM-R) individuals also known as “Resistors”) will also be included. Study of the DM-R population using KPMP protocols offers a unique opportunity to identify protective factors against complications of diabetes mellitus.
AKI
- The focus will be on acute intrinsic non-glomerular disease, primarily on acute tubular necrosis (ATN).
Inclusion / Exclusion Criteria
Inclusion:
- Diabetic Kidney Disease (Type 1 or 2)
- Hypertensive Kidney Disease
- Acute Kidney Injury
- Patient has had diabetes for many years but no clinical indications of CKD
Exclusion:
- Age (under 18)
- Glomerular disease, kidney transplant, malignancy, pregnancy
- Increased biopsy complication risk
For more specific details regarding inclusion and exclusion criteria for our various study populations, see the full clinical protocol on the KPMP website.
Cite KPMP in your publications
See: Citing KPMP data
ENROLLMENT VS PRIMARY ADJUDICATED CATEGORY
GOAL:
This document outlines the categorization of KPMP participants following enrollment and adjudication.
The goal of the KPMP Adjudication Committee is to review participants enrolled in KPMP and classify them into kidney disease categories that are considered the primary cause of the participant’s kidney disease based on clinical and pathological data. Additional causes noted in adjudication are available in the Core Clinical File and can be made available with a signed Data Use Agreement (DUA).
CATEGORIZATION:
Each participant is sub-categorized in different cohorts or disease classes at different steps of assessment
- Enrollment categories - participants enter the study classified in the following broad categories prior to kidney biopsy. Recruitment site investigators and research coordinators use study inclusion/exclusion criteria to determine the participant’s enrollment category. Refer to the KPMP protocol for more details on inclusion criteria.
- Healthy Reference: participant donating a kidney or undergoing treatment for kidney stones
- Chronic Kidney Disease (CKD): diagnosis of diabetes OR hypertension (based on inclusion criteria) AND evidence of persistent kidney damage (based on inclusion criteria)
- Acute Kidney Injury (AKI): evidence of acute kidney injury (based on inclusion criteria)
- Diabetes Mellitus Resilience (DM-R): participant has had diabetes for more than 25 years and has no clinical signs of kidney disease (based on inclusion criteria)
- Primary Adjudicated categories - upon review of the clinical and pathologic characteristics, participants are categorized using primary and secondary disease categories. The primary disease category reflects a consensus of pathologists and nephrologists on the primary cause of the participant’s kidney disease. The Primary Adjudicated categories are the following:
- Acute Interstitial Nephritis: inflammatory infiltration into the tubulointerstitium considered to be caused by drug exposure or other etiologies
- Acute Tubular Injury: clinical and histologic features of acute tubular injury
- Cannot be determined: the pattern of injury did not fit any known kidney disease agreed upon by the adjudication committee or the predominant pattern of injury could not be determined amongst several plausible underlying kidney diseases, or there was not enough information (clinical or limited tissue sample)
- Diabetic Kidney Disease: histologic features consistent with diabetic kidney disease in an individual with a history of diabetes
- Hypertensive Kidney Disease: histologic features consistent with hypertensive kidney disease in an individual with a history of hypertension
- Other: another kidney condition, including IgA nephropathy, focal segmental glomerulosclerosis, membranous nephropathy, fibrillary glomerulonephritis, etc. More specific diagnoses are available for release with a DUA.
ADJUDICATION PROCESS:
The Adjudication Team at KPMP comprises a group of Pathologists and Clinicians. Each participant’s case is reviewed with the following information:
- Whole Slide Images in Digital Pathology Repository (DPR)
- Clinical Packet: REDCap participant reported medical history, medications, and lab data completed by the recruitment site
- Pathology Packet: Diagnostic core disease category assessment REDCap form
- Electron Microscopy (EM) Images
- Recruitment Site Pathologist generated pathology report
Upon review of the materials, the KPMP adjudicators (clinicians and pathologists) meet and reach a consensus of primary and secondary categories and record in KPMP’s REDCAP Adjudication form.
Data is available in our Core Clinical File and is accessible with a DUA.
ENROLLMENT VS. PRIMARY ADJUDICATION:
The KPMP Enrollment Category reflects how each participant is enrolled in the KPMP study prior to biopsy based on inclusion criteria. For more details on the KPMP study criteria, please visit our Clinician Resources page. KPMP biopsies of participants enrolled in the CKD, AKI, or DM-R cohorts (“Enrollment categories”) are adjudicated through a comprehensive review of the participant’s clinical history and kidney biopsy pathology by expert nephrologists and renal pathologists on a consensus basis (“Primary Adjudicated categories”). Enrollment category and Primary Adjudicated category may differ.
For example, take a participant presenting with AKI enrolled in the AKI enrollment category. Upon adjudication:
- Clinical history reveals a long-standing history of hypertension and diabetes.
- Pathology review shows significant diabetic, global, and segmental glomerulosclerosis and severe arteriolosclerosis with moderate arteriolar hyalinosis. While mild to moderate tubular and interstitial injury in this case is present, and therefore the enrollment category is correct, the chronic damage and long-standing clinical history are more notable. The adjudication team ascertains that the primary driver of this participant’s kidney disease is diabetic kidney disease.
- This participant would have an Enrollment category of AKI and a Primary Adjudicated category of Diabetic Kidney Disease.
This adjudication process ensures a more accurate classification of kidney disease by integrating both clinical and pathologic insights, which is crucial for research, treatment decisions, and understanding disease progression.
Figure 1 demonstrates that the Enrollment category and Primary Adjudicated category do not always align.

Biopsy Processing and Distribution
The goal for KPMP ideally is to obtain 3 cores from each participant, which allows for multiple -omics technologies to be run on the same participant (though on different core samples). Core 1 is used for diagnostic purposes; Cores 2 and 3 are solely used for research:

For more information about the current triage process for the biopsy cores, see the pathology protocol on the KPMP website.
KPMP Data Types
Clinical data
A wide variety of clinical phenotype data is being collected about each participant in the KPMP, including longitudinal follow-up data. The KPMP REDCap codebook outlines all of the collected clinical phenotype data. View the REDCap Codebook.
KPMP has created a baseline clinical phenotyping data file that allows for a faster turnaround time for clinical data requests once a Data Use Agreement (DUA) has been completed. This file can be sent to users both internally and externally with DUAs. Variables include sex, age, race, disease type, condition, KDIGO stage, duration, comorbidities, family and personal history, frequently requested lab values, and medications. To find out more about the contents of this baseline clinical file, view the Baseline Clinical Phenotyping Data Dictionary.
Pathology data
Whole slide images
KPMP biopsy tissue is processed for light microscopy, immunofluorescence microscopy, and electron microscopy. Standard light microscopic stains for the KPMP tissue are: H&E, PAS, trichrome, and Jones' silver. The light microscopy whole slide images are available for download in the Atlas Repository. See: KPMP Pathology Manual of Procedures for more details on the pathology workflow.
Pathology descriptor scoring
The Kidney Precision Medicine Project (KPMP) tubulointerstitial and vascular (TIV) descriptor scoring parameters were developed based on the NEPTUNE Digital Pathology Scoring System.1 This form includes 64 unique TIV descriptors, of which 10 are scored as percentages observed across the cortex and medulla, while the remaining descriptors are assessed categorically within the cortex or medulla. Two KPMP pathologists—a primary scorer and a quality control (QC) scorer—review and score all stained sections (H&E, PAS, trichrome, and Jones silver histochemical stains) scanned into whole-slide images and stored in the KPMP Digital Pathology Repository, following the KPMP Manual of Operating Procedures. Scoring discrepancies between the primary and QC pathologists are discussed and resolved through adjudication, with a third pathologist consulted if necessary. These descriptors provide a comprehensive list of non-glomerular structural and cellular abnormalities observed in these regions.
Pathology Descriptor Scoring data can be found in the Atlas Repository and is updated as new scores are made available.
Reference
- Barisoni L, Troost JP, Nast C, Bagnasco S, Avila-Casado C, Hodgin J, Palmer M, Rosenberg A, Gasim A, Liensziewski C, Merlino L, Chien HP, Chang A, Meehan SM, Gaut J, Song P, Holzman L, Gibson D, Kretzler M, Gillespie BW, Hewitt SM. Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol. 2016 Jul;29(7):671-84. doi: 10.1038/modpathol.2016.58. Epub 2016 Apr 22. PMID: 27102348; PMCID: PMC5515468.
Molecular data
Several tissue interrogation technologies are active in the KPMP. To maximize impact of the limited kidney tissue, participating sites are required to submit their technology for a review of feasibility, reproducibility, rigor, and value of the technology to achieve the goals of the KPMP. The table below lists all of the omics and imaging technologies that are approved and actively analyzing kidney biopsy tissue. The multimodal techniques applied on KPMP biopsies are expected to evolve over time.
To learn more about each tissue interrogation approach, see the Technologies section.
See all available data in KPMP
Approved tissue interrogation technologies
Metadata standards
The Kidney Precision Medicine Project is generating a large amount of data. To enable data accessibility and interoperability, each dataset that is generated must be accompanied by a standard set of metadata. The required metadata properties vary by technology. For the metadata properties being collected for all of the KPMP technologies, refer to the Metadata page to see all of the metadata templates.
How to cite data used from the KPMP
The following citation must be used when citing KPMP data. KPMP follows the AMA standard for its citations.
The results here are in whole or part based upon data generated by the Kidney Precision Medicine Project. Accessed Month Day, Year. https://www.kpmp.org.
The Kidney Precision Medicine Project (KPMP) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through the following grants: U01DK133081, U01DK133091, U01DK133092, U01DK133093, U01DK133095, U01DK133097, U01DK114866, U01DK114908, U01DK133090, U01DK133113, U01DK133766, U01DK133768, U01DK114907, U01DK114920, U01DK114923, U01DK114933, U24DK114886, UH3DK114926, UH3DK114861, UH3DK114915, and UH3DK114937. We gratefully acknowledge the essential contributions of our patient participants and the support of the American public through their tax dollars.
NOTE: Remember to replace "Accessed Month Day, Year" with the correct specific details.

